Gene: [0Xq271/F9] coagulation factor IX (plasma thromboplastic component); hemophilia B (Christmas disease; coagulation factor IX deficiency);
|
FUN |
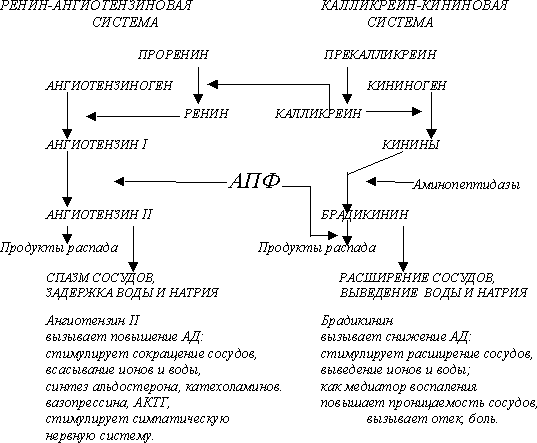
[1] Converts coagulation factor X to Xa.
[2] Systematic name: not known. [3] Catalyzes hydrolysis one arg-|-ile bond in factor X to form factor Xa." |
|
MOP |
The molecular product is a serum glycoprotein (MM 56 kD). It is synthesized by hepatocytes as a single-strand polypeptide. N-terminal region of the primary translation product contains 46 amino acids and two domains: the signal peptide (28 amino acids), which is necessary for transferring the protein through membrane, and the (Thr...Arg) propeptide (18 amino acids), which serves for post-translational modification and (PTM) and the consequent secretion of inactive F(IX) into the blood flow. The PTM occurs after the splitting of the signal peptide and includes the vitamin K-dependent gamma-carboxylation of the first 12 Glu in the catalytical peptide, beta-carboxylation of Asp in position <64>, and propeptide splitting. The mature enzyme consists of two peptide chains, light and heavy (L and H) ones, which arises from the splitting of the inactive catalytic polypeptide." |
|
MUT |
The mutant forms include F(IX)-Chapel Hill (substitution of Arg
for Hys at position 145 (Noyes-1983), that prevents the peptide
splitting and hence the enzyme activation), F(IX)-Alabama
(substitution of Asp for Gly at position 47 (Davis-1984)), 'splicing'
mutations (see Rees-1985), large deletions (see Giannelli-1983 and
Hassan-1985), and F(IX)-Campbridge (substitution of Arg (codon ag |
|
REF |
MUT "Bentley AK &: Cell, 45, (May), 343-348, 1986 LOC "Boyd &: Ann Hum Genet, 48, 145-152, 1984 LOC,POL "Camerino &: PNAS, 81, 498-502, 1984 CLO,SEQ "Choo KH &: Nature, 299, (9 Sep), 178-180, 1982 POL,PND "Connor &: J Med Genet, 22, 441-446, 1985 MUT "Davis &: Blood, 64, (Suppl), 262, 1984 MUT,POG "de la Salle C &: Clin Genet, 38, N6, 434-440, 1990 MUT "Diuguid &: PNAS, 83, N16, 5803-5807, 1986 POL,PND "Giannelli F &: Lancet, 1, N8371, 239-241, 1984 MUT "Giannelli F &: Nature, 303, (12 May), 181-182, 1983 MUT "Hassan &: Blood, 66, N3, 728-730, 1985 POL,PND "Hay CW &: Blood, 67, N5, 1508-1511, 1986 SEQ,POL "Jagadeeswaran P &: Somat Cell Mol Genet, 10, N5, 465-473, 1984 SEQ,PEP "Kurachi K, Davie: PNAS, 79, 6461-6464, 1982 POL,PND "Lillicrap &: Brit J Haematol, 62, 557-565, 1986 POL,PND "Lubahn DB &: AJHG, 40, 527-536, 1987 POL,PND "Mulligan L &: Hum Genet, 75, 381-383, 1987 EXP,PND "Nisen &: New Engl J Med, 315, N18, 1139-1142, 1986 MUT "Noyes CM &: PNAS, 80, 4200-4202, 1983 LOC "Purrello M &: EMBO J, 4, 725-729, 1985 LOC "Quirk S &: CCG, 39, 121-124, 1985 MOP,EXP,EVO "Rees DJ &: EMBO J, 7, N7, 2053-2061, 1988 MUT "Rees DJ &: Nature, 316, 643-645, 1985 EVO,MAM,GEN,MOP,SEQ "Sarkar &: Genomics, 6, N1, 133-143, 1990 LOC "Schwartz &: Hum Genet, 76, 64-67, 1987 ENG,EXP "Simpson PJ &: Gene, 61, 373-383, 1987 |
|
KEY |
hem, clot |
|
CLA |
coding, basic |
|
LOC |
0X q27.1 |
|
MIM |
MIM: 306900 |
|
EZN |
ENZYME: 3.4.21.22 |
Смотрите также: